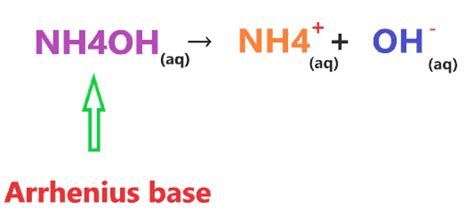
NH4OH is a weak base because it partially dissociates into NH+ and OH- ions in aqueous solutions and the amount of OH- ions produced is low.
In this regard, is nh4oh a weak or strong base?
NH4OH, a weak base when it has more concentration of H ions than OH ions, because for a strong base more number OH ions are required not H ions, is a colorless solution, which weighs 35.046 g/mol.
Additionally, is nh4oh a base or acid? Answer and Explanation: The formula NH4OH is ammonium hydroxide. It is a solution that is made up of ammonia and water. The pH of NH4OH is 11.6, so it is a base.
Keeping this in consideration, is ammonium hydroxide a weak base?
👉 For more insights, check out this resource.
Ammonium hydroxide is a weak base because it does not dissociate completely into ions. To determine if a chemical compound is a base, look at the
Is LiOH strong or weak?
👉 Discover more in this in-depth guide.
LiOH is a weak base because it is more covalent due to small size. So, they cannot yield hydroxyl ions easily in aqueous solutions which is the main cause of basicity. Why is the reaction between a weak acid and a strong base resulting in a pH above 7?
Is NaOH a strong Nucleophile?
Take a species like NaOH. It's both a strong base and a good nucleophile. When it's forming a bond to hydrogen (in an elimination reaction, for instance), we say it's acting as a base. Similarly, when it's forming a bond to carbon (as in a substitution reaction) we say it's acting as a nucleophile.
Is NH4Cl a strong or weak acid or base?
As mentioned in the other answer, NH4Cl is an “acidic” salt, formed by the neutralization of a strong acid (HCl) with a weak base (NH3). Therefore, when the salt is completely dissociated in an aqueous solution, it forms NH4+ and Cl- ions. Explanation: The solution of Ammonium Chloride is therefore acidic.
Is HCl a strong base?
Hydrogen chloride (HCl) ionizes completely into hydrogen ions and chloride ions in water. A weak acid is an acid that ionizes only slightly in an aqueous solution. Weak acids, like strong acids, ionize to yield the H+ ion and a conjugate base. Because HCl is a strong acid, its conjugate base (Cl−) is extremely weak.
Is koh a weak base?
KOH is potassium hydroxide. Since it is composed of the hydroxide anion (OH-), it is a strong base. In solution, the hydroxide anion will completely react with any available protons, that is why KOH is a strong base. It is not an acid of any type, weak or strong, since KOH does not contribute any protons to solution.
Is NaCl an acid or base?
NaCl is formed by the reaction of HCl and NaOH. Both are strong acids and bases. When a strong acid and a strong base react together the resultant is salt and water. Therefore NaCl is a salt.
Is NaCl an electrolyte?
NaCl is an ionic compound. Since it dissolves completely forming Na^+ and Cl^- ions it is classified as a strong electrolyte. Strong electrolytes are completely dissociated into ions in solution and conduct an electrical current strongly that is, they completely ionize in water and are good conductors of electricity.
Which is stronger NaOH or nh4oh?
Sodium Hydroxide is a stronger than Ammonium hydroxide because Sodium Hydroxide fully dissociate when dissolved in water where is Ammonium hydroxide Vaishali dissociates when dissolved in water.
What are the examples of weak bases?
By this frame of reference, some examples of weak bases are ammonium hydroxide (NH4OH), hydrazine (N2H4), somewhat structurally related with the anterior one), phosphine (PH3), zinc hydroxide [Zn(OH)2] (besides the fact that this hydroxide is an anphoteric one) and, amongst the organic compounds, there is much more
Which hydroxide is a weak base?
For example, acetic acid (HC2H3O2) and oxalic acid (H2C2O4) are weak acids, while iron hydroxide, Fe(OH)3, and ammonium hydroxide, NH4OH (which is actually just ammonia, NH3, dissolved in water), are examples of weak bases.
What are the strong bases?
Strong bases are able to completely dissociate in water
- LiOH - lithium hydroxide.
- NaOH - sodium hydroxide.
- KOH - potassium hydroxide.
- RbOH - rubidium hydroxide.
- CsOH - cesium hydroxide.
- *Ca(OH)2 - calcium hydroxide.
- *Sr(OH)2 - strontium hydroxide.
- *Ba(OH)2 - barium hydroxide.
Which is the weakest base?
Weak Acids & Bases
| Common Weak Acids | Common Weak Bases |
| Formic | HCOOH | ammonia |
| Acetic | CH3COOH | trimethyl ammonia |
| Trichloroacetic | CCl3COOH | pyridine |
| Hydrofluoric | HF | ammonium hydroxide |
Why is I a weak base?
Since bases are proton acceptors, the base receives a hydrogen ion from water, H2O, and the remaining H+ concentration in the solution determines pH. Weak bases will have a higher H+ concentration because they are less completely protonated than stronger bases and, therefore, more hydrogen ions remain in the solution.
What is strong base and weak base with example?
A strong base is a base that is completely dissociated in an aqueous solution. These compounds ionize in water to yield one or more hydroxide ion (OH-) per molecule of base. In contrast, a weak base only partially dissociates into its ions in water. Ammonia is a good example of a weak base.
Which ion is the strongest base?
If the conjugate acid is a weak acid (high pKa), then the anion is a strong base. The conjugate acid with the highest pKa is methane (CH4) it is the weakest acid of the group. Therefore the CH3− in MeLi is the strongest base in the group.
Is ammonium hydroxide a base or alkali?
Ammonium hydroxide can either exist as NH3 and H2O or as NH4+ and OH- through a reversible reaction. When ammonium hydroxide is allowed to reach equilibrium, far more NH3 and H2O exists compared to NH4+ and OH-, so the concentration of OH- is low and hence ammonium hydroxide is considered to be a weak alkali.
Is MgCl2 an acid or base?
MgCl2 is a salt of a strong acid and a strong base, therefore it is neutral salt.
Is ch3nh2 a strong or weak base?
Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. Methylamine is a good nucleophile as it is an unhindered amine. As an amine it is considered a weak base.
Posting Komentar untuk "Is nh4oh a weak base?"